
What is hydrofluoric acid, and what are the dangers ?
Hydrofluoric acid is a corrosive chemical whose formula is HF. It is volatile, colourless and has an acrid odour. Anhydrous, it is gaseous at ambient temperatures and may be dissolved in water up to a concentration of 75%. In an aqueous solution, hydrofluoric acid dissociates, meaning that it forms ions of H+ acids and F– ions called fluorides.
According to the harmonised classification of chemicals, hydrofluoric acid is classified as fatal in contact with skin (H310), fatal if swallowed (H300), fatal if inhaled (H330) and causes severe skin burns and eye damage (H314).
Diluted solutions of hydrofluoric acid are also classified as hazardous when concentrations are higher than 0.1%. Solutions of hydrofluoric acids corrode most metals and release dihydrogen, a gas that is flammable and explosive.
A few common uses
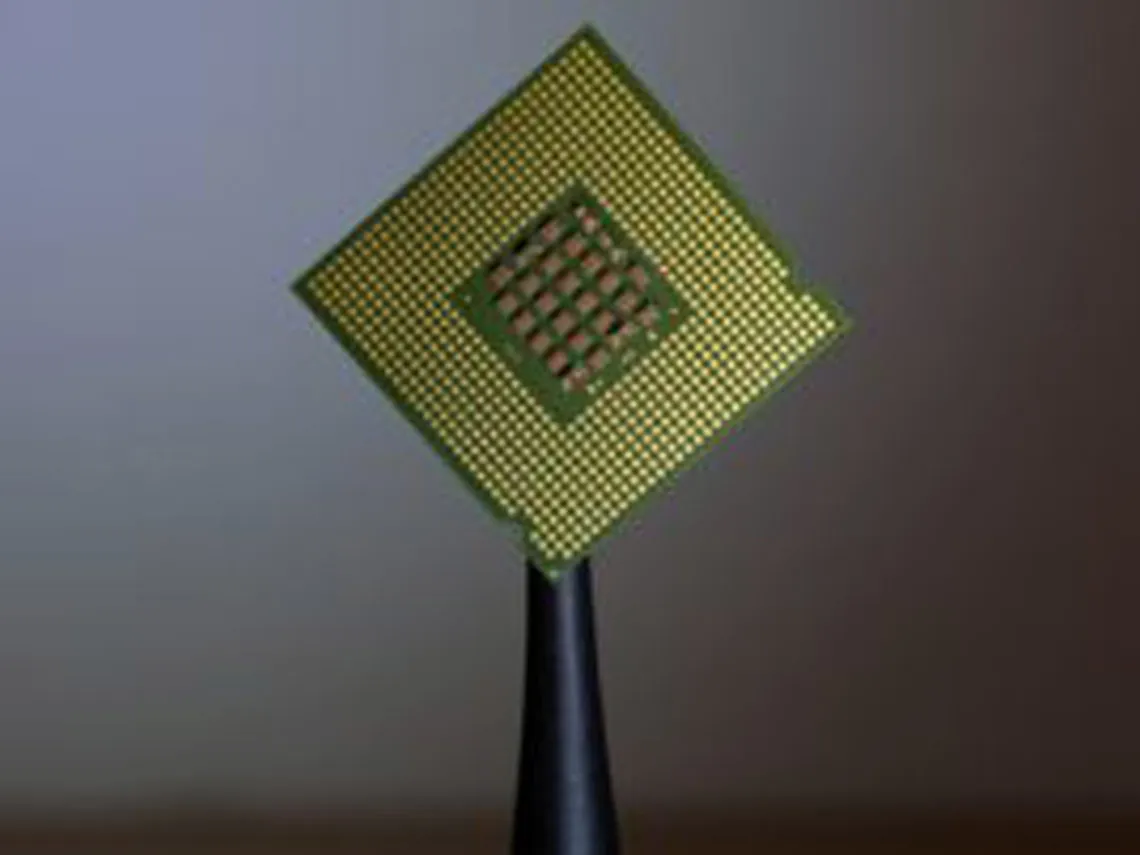
Hydrofluoric acid is capable of dissolving silica, which is why it’s used in many operations for etching, cutting glass, in refining, or in semi-conductor manufacturing.

Low concentrations are also found in everyday products used in mechanics, in particular in the form of stripping pastes used after welding work, to clean car rims, pickling operations, etc.
How does hydrofluoric acid affect the organism ?
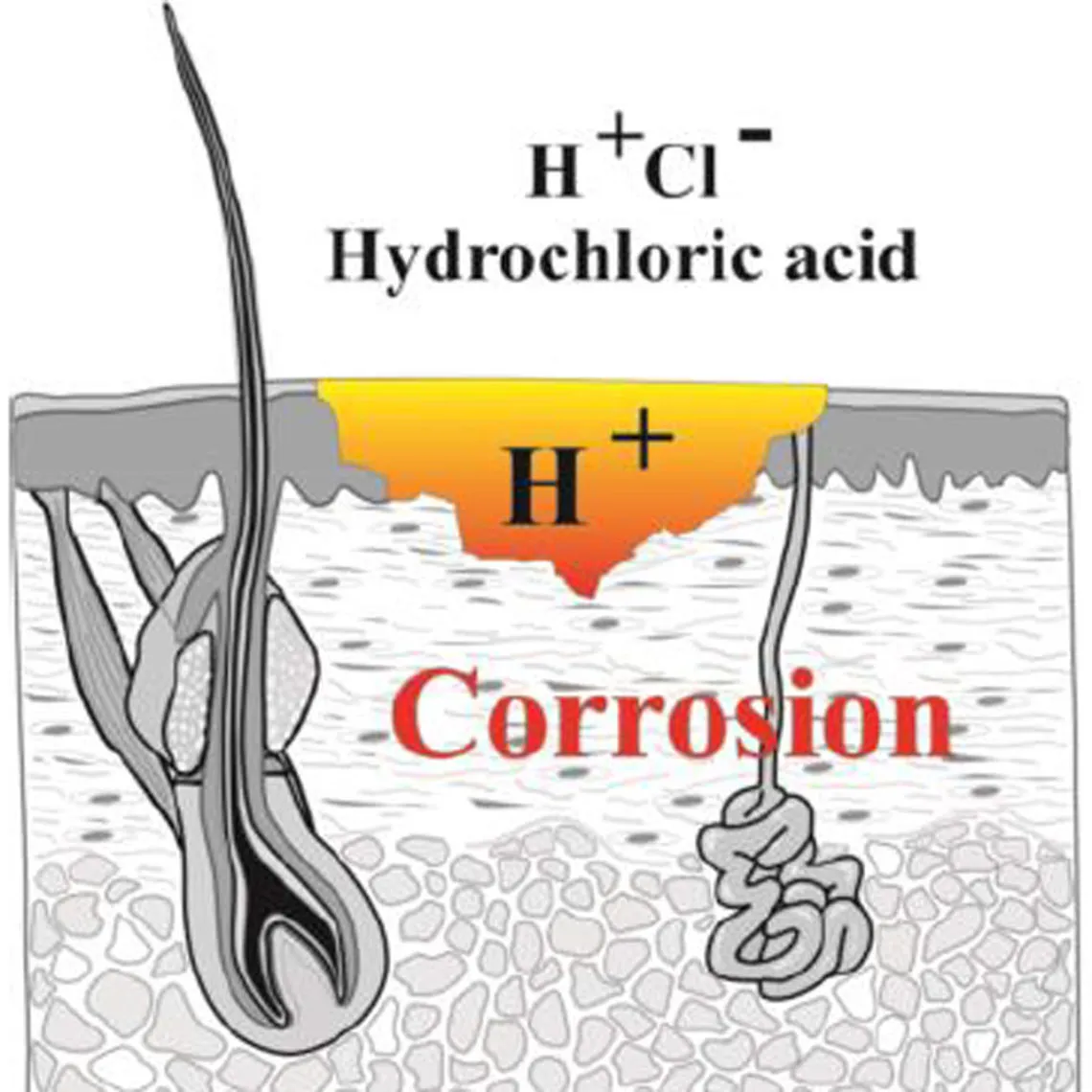
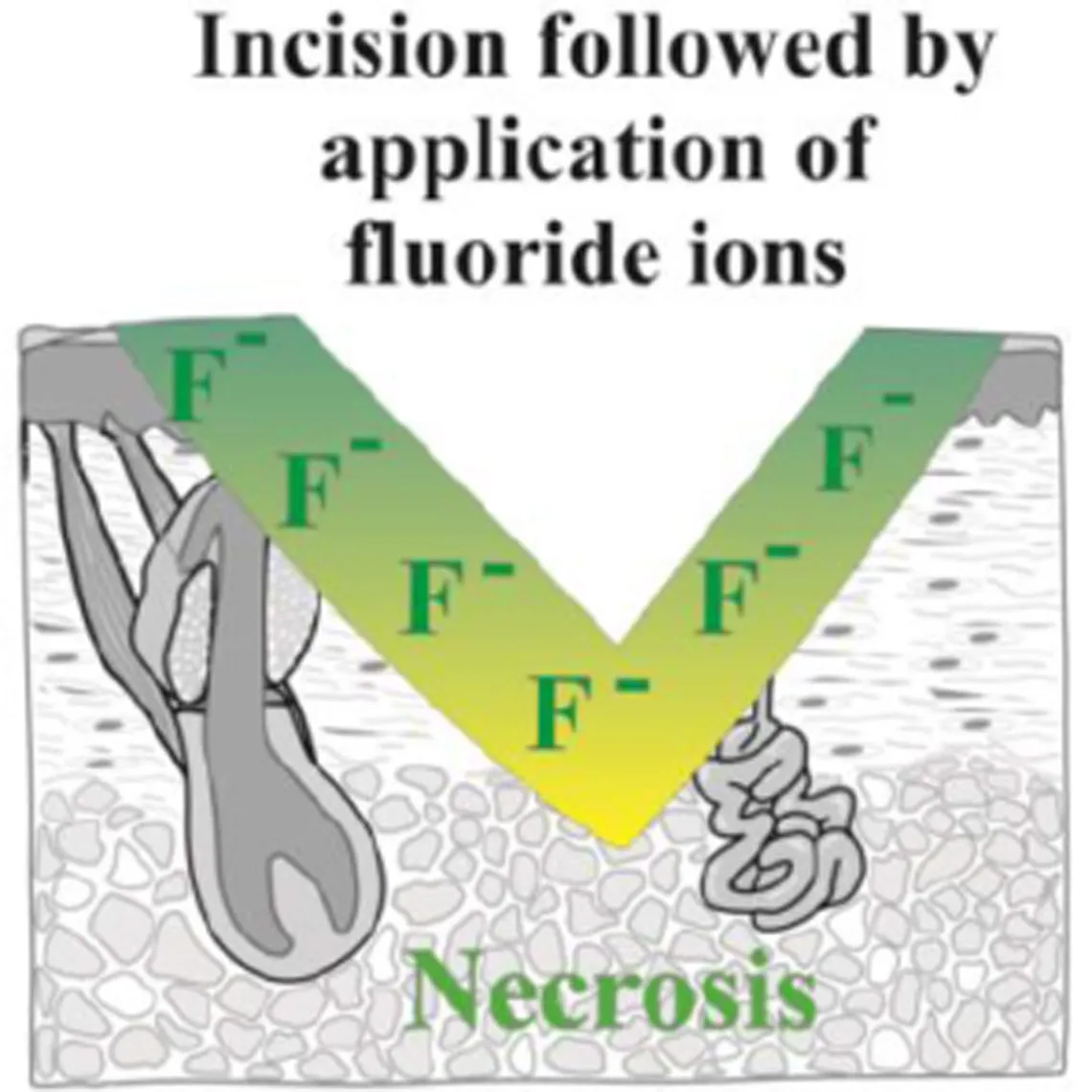
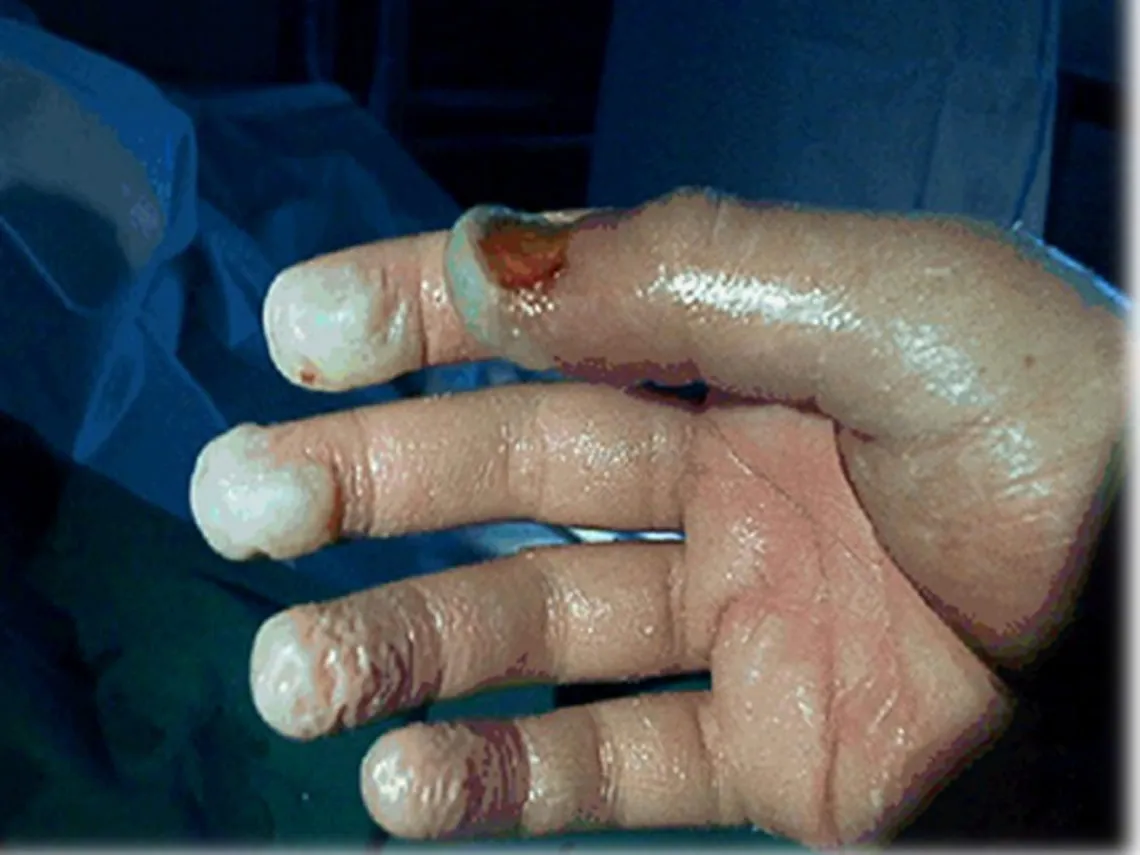
→ This is why it is very important to protect oneself from all contact with hydrofluoric acid or its derivatives even if the concentrations or volumes used are low.
How can you limit your employees’ exposure ?

Beforehand :
- Limit your stock of HF, use a substitute when possible and check that it cannot come into contact with incompatible chemicals (water, strong bases, etc.)
- Check whether the materials used are compatible with HF.
- Use collective protective equipment (chemical hoods, closed circuits, etc.) and wear mandatory, appropriate personal protective equipment
- Train personnel on the chemical hazards related to hydrofluoric acid.

On a daily basis or during maintenance :
To limit employee exposure to hydrofluoric acid or its derivatives, chemical decontamination and cleaning is advised for all surfaces where projections or drops of HF may have landed: lab tables, PPE, rims once treated, workstations, etc.
SAFUREX®, the chemical decontaminant, was created for decontaminating equipment that comes into contact with HF and fluorides. It neutralises the acid nature of the H+ ions by bringing the pH to a neutral pH, but also fixes the F– fluoride ions.
Once they’ve been fixed by SAFUREX®, the fluoride ions are no longer able to combine with the calcium or magnesium in the organism and are thus rendered harmless.
First Aid – Managing an accident :
In the event of hydrofluoric acid chemical projections, our recommendation is to decontaminate the victim as quickly as possible, using Hexafluorine® solution. Find out more on this first aid solution at Hexafluorine® solution
Calcium gluconate is often prescribed to provide a source of calcium to the organism and offset the effect of the fluorides.

In the event of spills :

The use of neutralising absorbents compatible with hydrofluoric acids, such as TRIVOREX® or ACICAPTAL®, allow spills to be neutralised safely. The acid nature of HF will be eliminated, emissions of acid vapours halted, thus minimising the hazard.
After handling the leak, it is important to decontaminate all equipment and PPE which have been contaminated by HF to avoid further contamination of staff.
To find out more :
- Annexe VI of EC REGULATION No. 1272/2008 FROM THE EUROPEAN PARLIAMENT AND THE COUNCIL
- For more information on manufacturing, transport, storage or selection of materials compatible with hydrofluoric acid: Eurofluor
- Examples of chemical accidents involving hydrofluoric acid: ARIA Flash from October 2015. Hydrofluoric acid, the worst of all acids?
- French National Research and Safety Institute (INRS) Factsheet on Hydrofluoric Acid