
What is crystalline silica?
Crystalline silica has a 3-dimensional structure that forms crystalline domains, which means that the presence of crystals can be observed on the microscopic level.
What differentiates it from amorphous silica or silicates ?
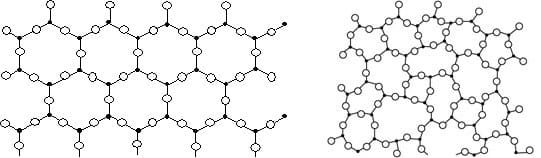
Silicates (sodium silicate, calcium silicate, magnesium silicate, potassium silicate, etc.) are derivatives of silica. The SiO2 groups are bound to other atoms, such as Al, Ca, Mg, K, etc. Thus they are not the same chemical entity and have different toxicity.
The special case of diatomaceous earth :
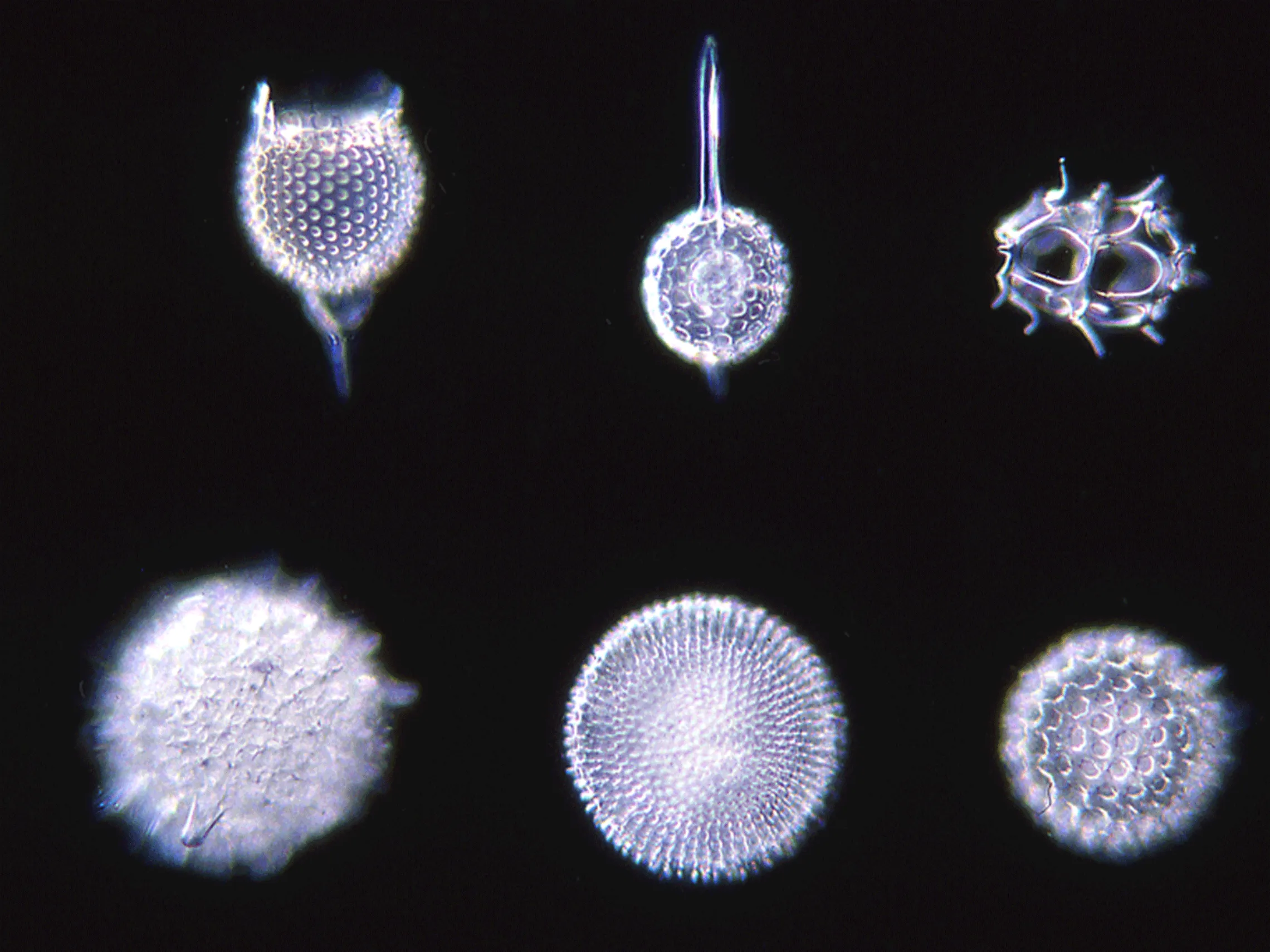
Diatomaceous earth produced naturally is mainly made up of amorphous silica, but can contain crystalline silica. There are different grades of diatomaceous earth that contain more or less crystalline silica, depending on the treatment applied:
Natural grade: Natural-quality products are dried at relatively low temperatures. These natural-quality products are mainly made up of amorphous silica, but they may contain up to 3% natural crystalline silica.1
Calcinated grade: Calcinated products are dried at higher temperatures, usually around 1000°C. Under the effects of such heat, part of the amorphous silica can undergo a crystallographic transformation to form crystalline silica, mainly in the form of cristobalite. Consequently, calcinated diatomite can contain up to 70% crystalline silica.2
Despite their attractive appearance and their natural origin, caution must be taken with diatomaceous earths as they can be a major source of crystalline silica.
Where can crystalline silica be found?
| Source | Amorphous Silica | Crystalline silica | |
|---|---|---|---|
| Natural | Mineral | / | α-Quartz |
| / | Quartz (CAS 14808-60-7) | ||
| / | Cristobalite (CAS 14464-46-1) | ||
| / | Tridymite (CAS 15468-32-3) | ||
| Biogenic | Diatomaceous earth (Kieselguhr, CAS 61790-53-2) Crystalline silica content <3% | ||
| Calcinated diatomaceous earth (CAS 91053-39-3) Crystalline silica content 0-40% | |||
| Flux-calcinated diatomaceous earth (CAS 68855-54-9) Crystalline silica content up to 70% | |||
| Synthetic | Synthesis | Fumed silica (CAS 112945-52-5) | / |
| Silica gel (CAS 112926-00-8) | / | ||
| Precipitated silica (CAS 112926-00-8) | / | ||
| Colloidal silica | / | ||
| Fused silica / Electric arc silica | / | ||
| By-product | Silica fumes (CAS 69012-64-2): partial transformation into cristobalite | ||
| Fused silica (CAS 60676-86-0) | |||
What impacts on health?
Exposure route to crystalline silica is mainly through inhalation. When used as an absorbent, granulates or filler in building materials, silica must first be ground or crushed. This is when fine particles of crystalline silica are formed.
These fine particles can then be easily inhaled, leading to certain diseases or disfunction of the body, the most serious being silicosis and lung cancer.
Chronic silicosis is a progressive fibrous lung disease that is potentially fatal and whose symptoms include respiratory insufficiency, shortness of breath, fatigue and chest pain. Silicosis is specifically the result of inhaling silica in its crystalline form. Lung transplant is the only treatment currently available. Silicosis can be recognized as an occupational disease.
Once silicosis has been diagnosed, the disease continues to progress even if there is no additional exposure.
Many studies on professional populations have shown a connection between inhaling crystalline silica and lung cancer. The IARC has concluded that there is enough proof of carcinogenicity in humans and animals for crystalline silica to be classified as carcinogenic for humans since 1997 (group 1).4
The mechanisms of carcinogenicity at work with particles of crystalline silica have not yet been identified with certainty. According to the IARC, the most likely hypothesis as to the mode of action is an indirect genotoxicity induced by the reduction in the lung alveoli’s capacity to eliminate particles of crystalline silicate, leading to persistent lung inflammation.
Other respiratory diseases:
Many studies have indicated that occupational exposure to crystalline silica is associated with non-malignant respiratory diseases other than silicosis. According to OSHA, respirable exposure to crystalline silica notably increases the risk of chronic bronchitis and an alteration of the respiratory functions.
The Regulatory Situation:
In France: Regulatory 8-hour Occupational Exposure Limit Values (OELV-8h) are set by the French Labour Code5 for the three forms of crystalline silica: for quartz at 0.1 mg.m-3, for cristobalite and tridymite at 0.05 mg.m-3.
In an opinion issued in April 2019, ANSES indicated that the OELV-8h of 0.1 mg.m3 is not protective enough and recommended revising these OELVs for crystalline silica given the health risks. A reduction in these OELVs can therefore be expected in the near future.
On the European level: So far, under the CLP regulation6, crystalline silica does not have harmonised classification and labelling. In fact, it was considered that work on this classification was not currently necessary as Directive 2017/2398 of the European Parliament and of the Council of 12 December 2017 had already classified “Work involving exposure to respirable crystalline silica dust generated by a work process” as carcinogenic.7
The work carried out by companies that produce diatomaceous earth has shown that labelling is desirable when fine particles of crystalline silica are present in a product8 in order to reflect the dangers of silicosis:
| Percentage of fine particules of cristalline silica | Labelling | Danger |
|---|---|---|
| Greater than or equal to 10% | Sto re 1 : Specific toxicity for lungs after long-term exposure | |
| Between 1 and 10% | Sto re 2 : Specific toxicity for lungs after long-term exposure | |
| Less than 1% | None | None |
USA: In 2016, OSHA (Occupational Safety and Health Administration) set an OELV-8h of 0.05 mg.m-3 for respirable crystalline silica and proposed the value of 0.025 mg.m-3 as the action value.
What protections are needed in spill management?


To manage common liquid spills such as oils, hydrocarbons, solvents, or aqueous liquids of little danger, we recommend using POLYCAPTOR® multipurpose absorbent.

To manage spills of corrosive chemicals such as strong acids or bases, we recommend using a neutralizing absorbent to eliminate chemical hazards such as TRIVOREX® neutralizing absorbent.
1 ANSES, D’après l’audition de Matériaux Industriels France par l’ANSES en 2016, 2019.
2 INRS 2007
3 INRS 2007
4 International Agency for Reseach on Cancer (IARC – CIRC en Français), Monographs Volume 100C.
5 Code du travail article R4412-149.
6 Règlement (CE) n o 1272/2008 du Parlement européen et du Conseil du 16 décembre 2008 relatif à la classification, à l’étiquetage et à l’emballage des substances et des mélanges.
7 Site web ECHA
8 I. International Diatomite Producers Association, A Guide to Safe Handling of Diatomaceous Earth products.